
The US FDA New Drug Approvals in March 2025
Shots:
-
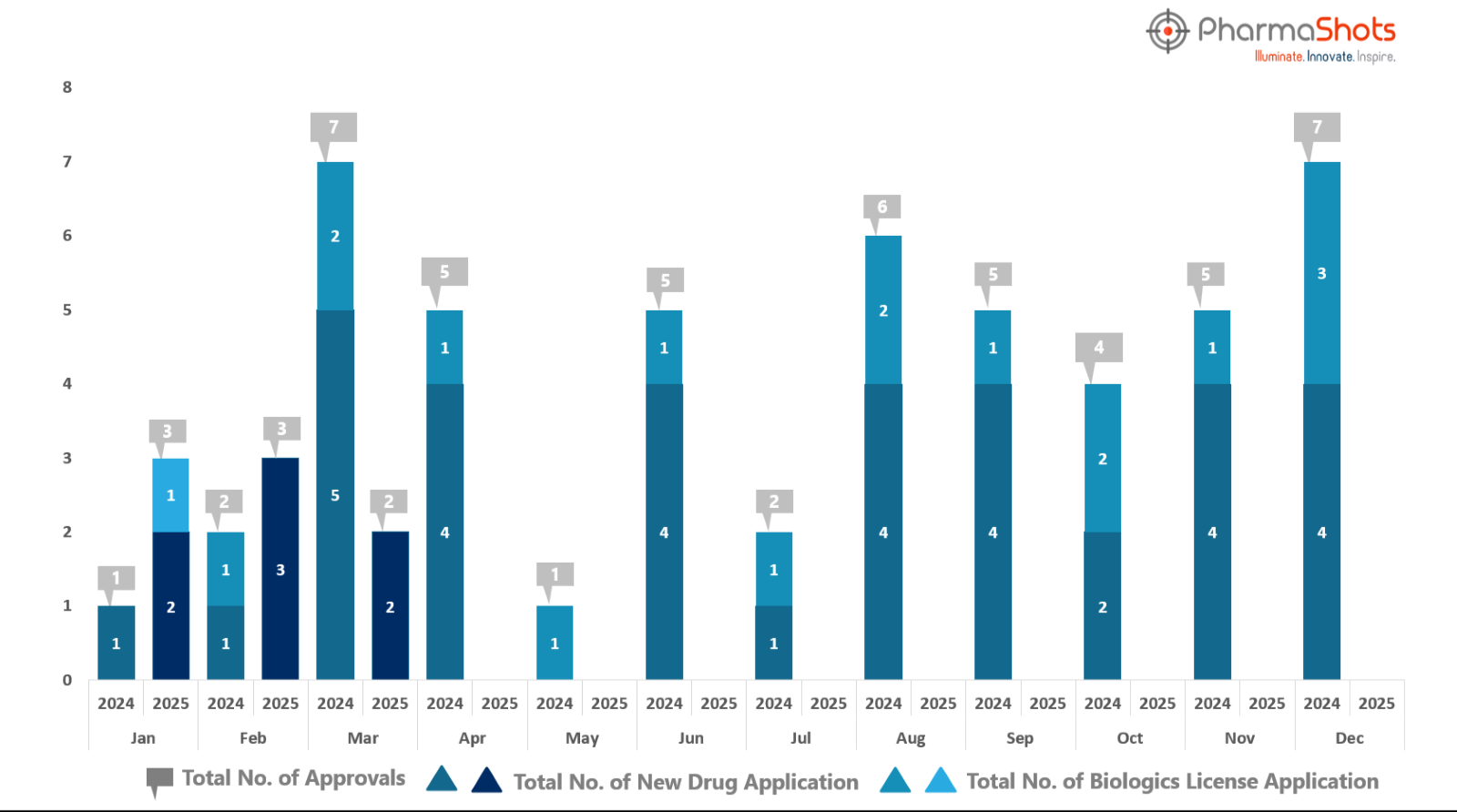
PharmaShots has compiled a list of US FDA-approved drugs in the month of March 2025
-
The US FDA has approved a total of 2 new drug including 2 new molecular entities leading to the treatment of patients and advances in the healthcare industry
-
The major highlighted drug was GSK’s Blujepa securing approval for treating Uncomplicated Urinary Tract Infections (uUTIs)
Company: GSK
Product: Blujepa
Active Ingredient: Gepotidacin
Disease: Uncomplicated Urinary Tract Infections (uUTIs)
Date: Mar 25, 2025
Shots:
-
The US FDA has approved Blujepa to treat uUTIs in female adults (≥40kg) & adolescents (≥12yrs., ≥40kg) caused by E. coli, K. pneumoniae, C. freundii complex, S. saprophyticus & E. faecalis; commercially available in H2’25
-
Approval was based on P-III (EAGLE-2, n=1531; EAGLE-3, n=1605) studies assessing gepotidacin (1500mg, PO, BID for 5 days) vs nitrofurantoin (100mg, PO, BID for 5 days) to treat uUTIs with the follow-up of 28 days
-
Studies depicted non-inferiority of gepotidacin to nitrofurantoin (SoC for uUTI), with the success rates of 50.6% (162/320) vs 47% (135/287; treatment difference: 4.3%) in EAGLE-2 & 58.5% (162/277) vs 43.6% (115/264; treatment difference: 14.6%) in EAGLE-3
Company: Alnylam Pharmaceuticals and Sanofi
Product: Qfitlia
Active Ingredient: Fitusiran
Disease: Hemophilia A or B
Date: Mar 28, 2025
Shots:
-
The US FDA has approved Qfitlia to treat routine prophylaxis and prevent or reduce the frequency of bleeding episodes in pts. (age≥ 12yrs.) with hemophilia A or B, with or without factor VIII or IX inhibitors. Regulatory submissions have been completed in China & Brazil
-
Qfitlia published clinical data in the NEJM in 2017, showing a reduction in bleeding rates in haemophilia pts. and initiating the P-III development program
-
In 2014, Sanofi gained global rights to co-develop & co-commercialize Qfitlia under a license & collaboration agreement which was later upgraded to full global rights in 2018, while Alnylam became eligible for tiered royalties of 15-30% on global net sales
Related Post: Insights+: The US FDA New Drug Approvals in February 2025
Tags

A passionate content writer with expertise in delivering high-quality and engaging content, Dipanshu is a keen reader and a versatile writer. Dipanshu dedicatedly covers news ranging from biopharma, life sciences, biotech, and MedTech to diagnostics and animal health companies, FDA, EMA, and biosimilar approvals. He can be contacted at connect@pharmashots.com














